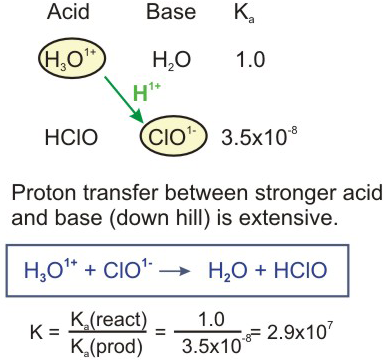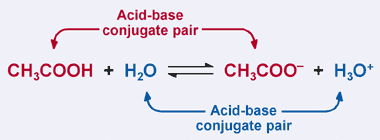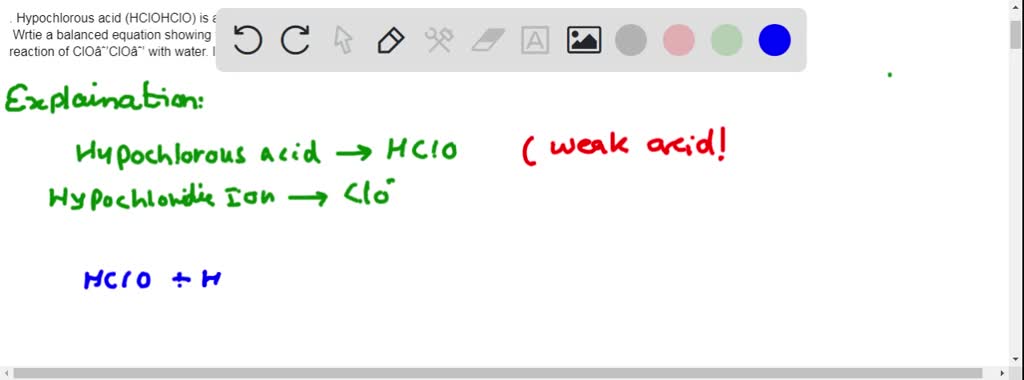
SOLVED: 1. Hypochlorous acid (HClOHClO) is a weak acid. The conjugate base of this acid is the hypochlorite ion (ClO−ClO−). Wrtie a balanced equation showing the reaction of HClOHClO with water. Include

SOLVED: Identify the conjugate acid or base of each of the following: You have 3 attempts Conjugate base of HNOz HNO+ NO: HNO: NOz Conjugate base of HCLO HzClO HCl HClO2 ClO

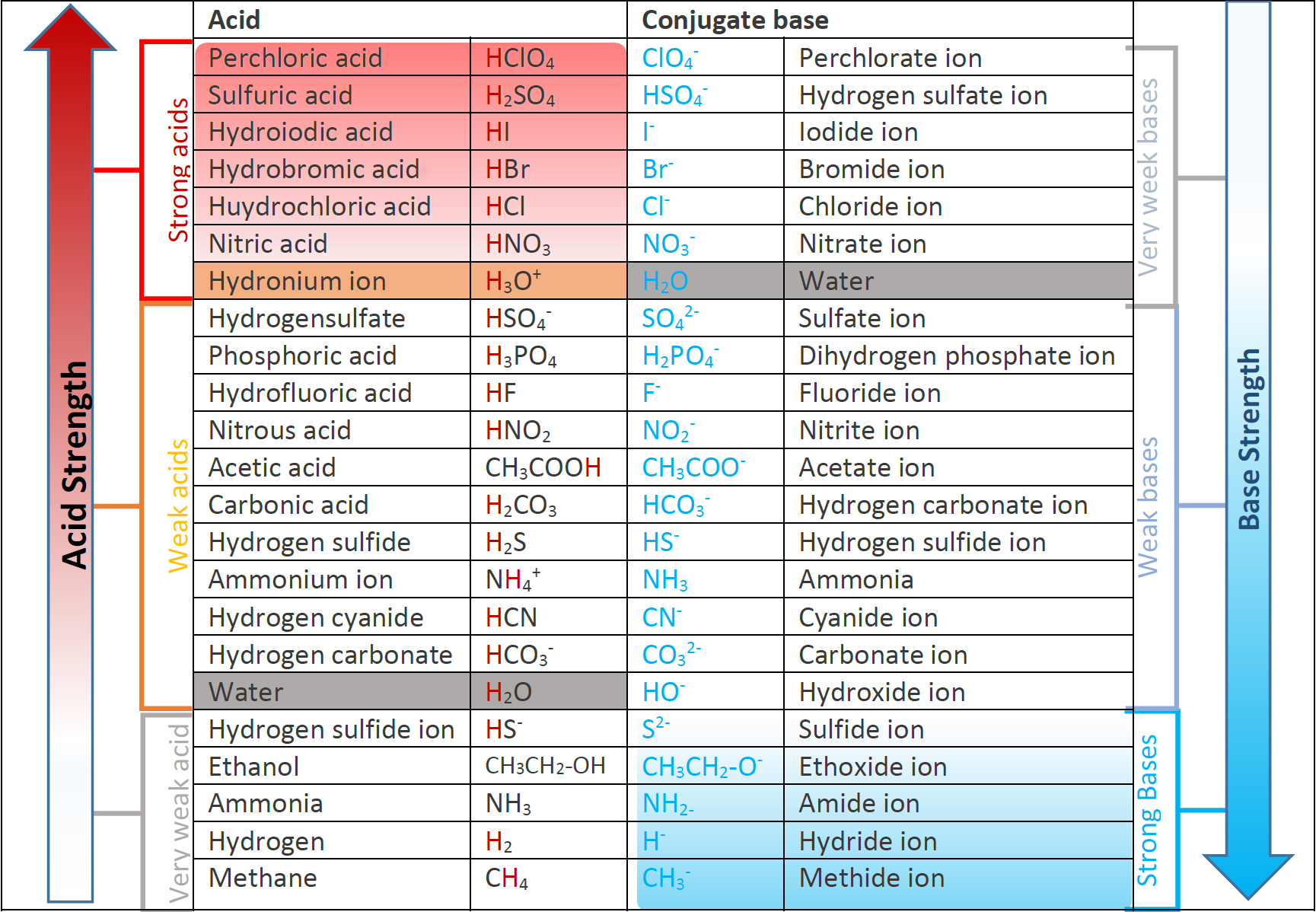
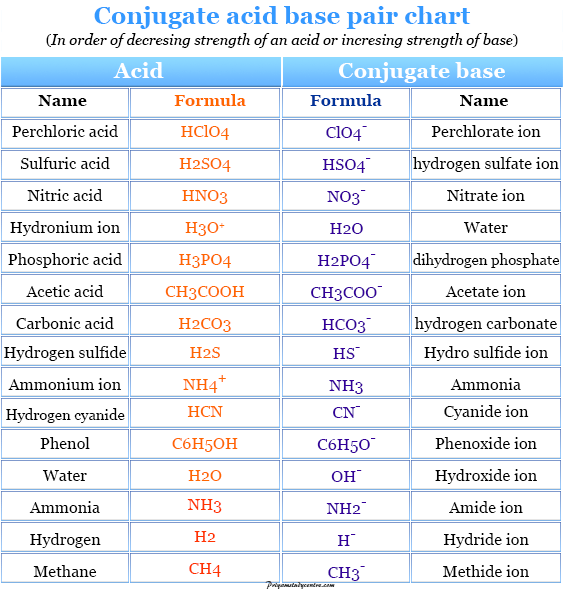
PDF) TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a (25 o C) HClO 4 ClO 4 - H 2 SO 4 HSO 4 - HCl Cl - HNO 3 NO 3 -

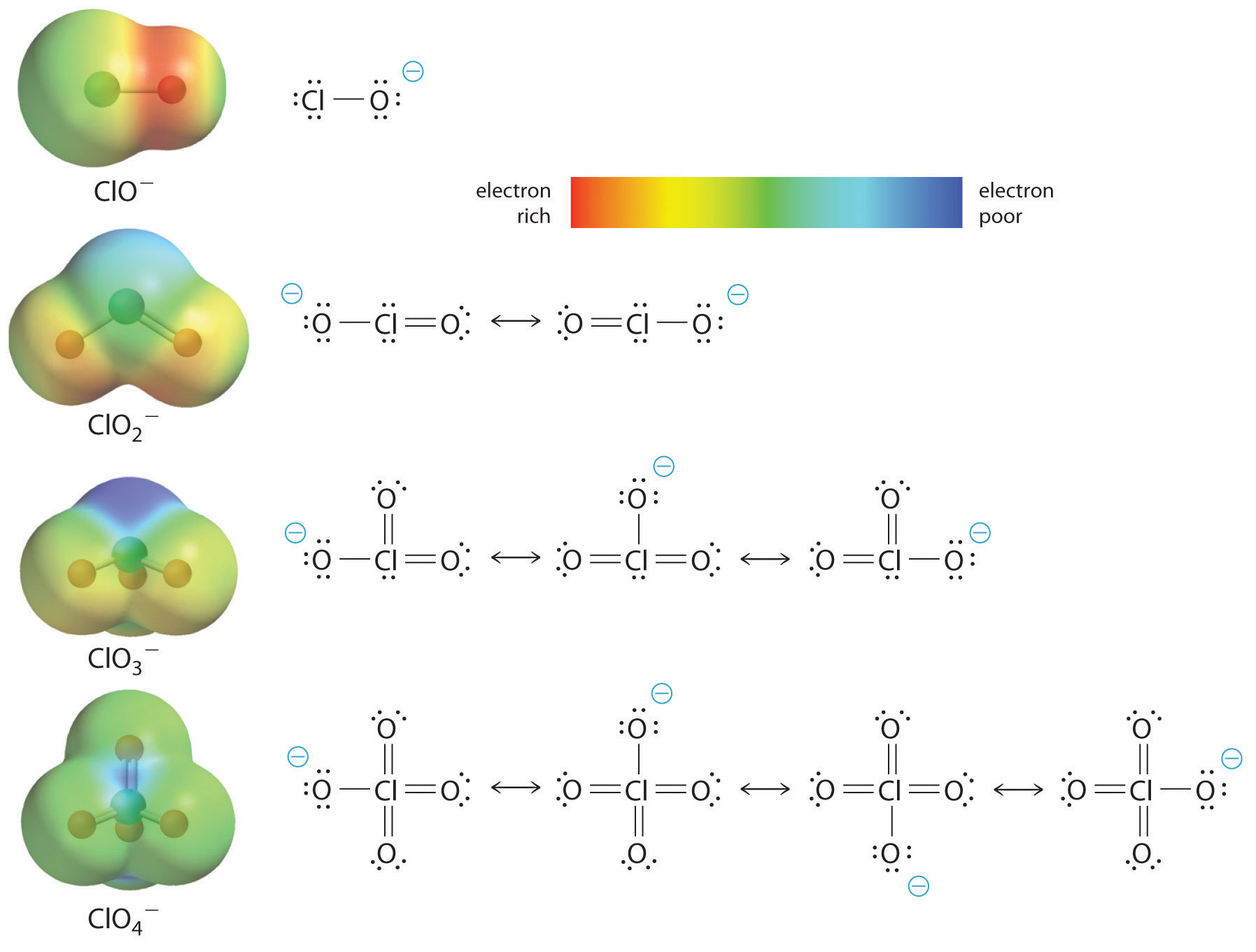
What is the strongest base, among the following? A. ClO^- B. ClO_2^- C. ClO_3^- D. ClO_4^- What is the weakest acid, among the following? A. HOI B. HOBr C. HOCl D. all

Reactions in Solution The most important substance on earth is water. In chemistry, water is necessary for many reactions to take place. Table salt (NaCl) - ppt download