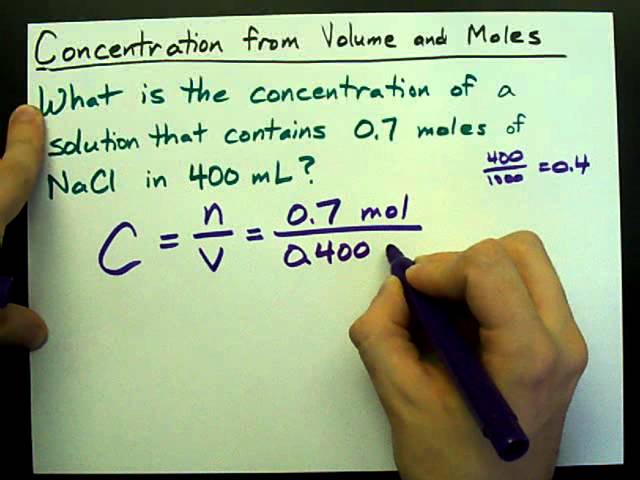SOLVED:Calculate the hydrogen ion concentration, in moles per liter, for solutions with each of the following pOH values. a. pOH=4.95 b. pOH=7.00 c. pOH=12.94 d. pOH=1.02
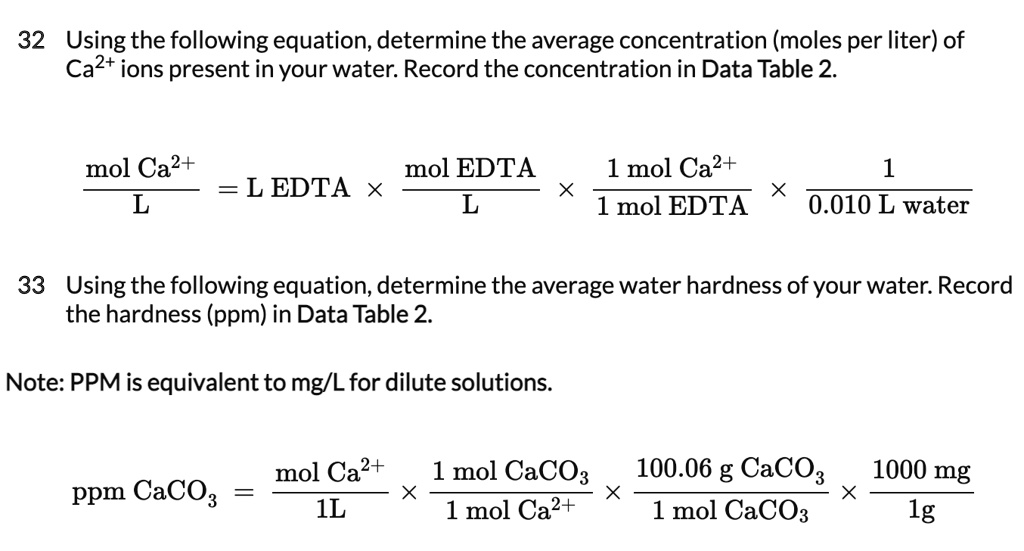
SOLVED: 32 Using the following equation, determine the average concentration (moles per liter) of Ca2+ ions present in your water: Record the concentration in Data Table 2- mol Ca2+ mol EDTA =
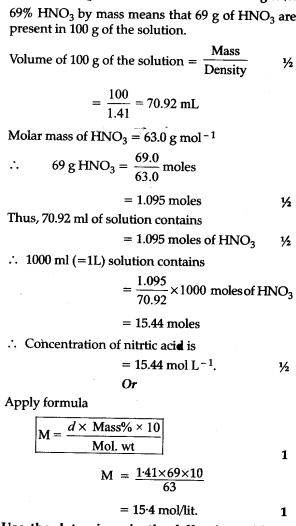
Calculate the concentration of nitric acid in moles per litre in a sample which has a density, 1.41 g ${{ml}^{-1}}$ and the mass percent of nitric acid in it being 69% -
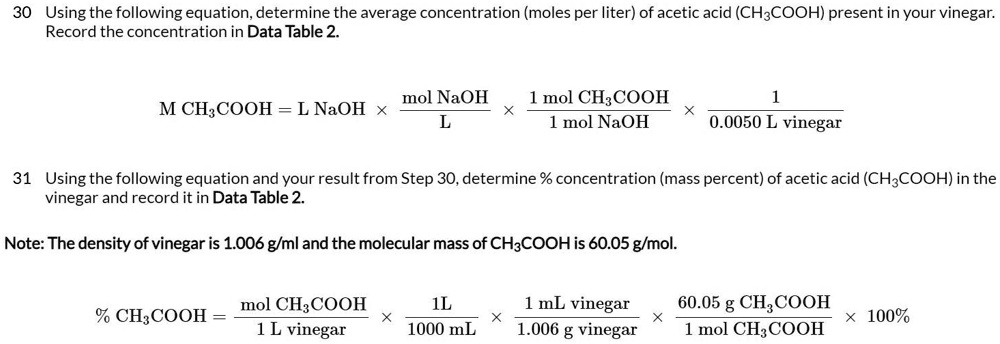
SOLVED: 30 Using the following equation; determine the average concentration (moles per liter) of acetic acid (CHzCOOH) present in your vinegar: Record the concentration in Data Table 2 mol NaOH mol CH:COOH

If the concentrations are expressed in mole litre^(-1) and time in sec then the units of the rate constant for the first order reaction are :

What is the approximate pH of a solution if the concentration of hydrogen ions is 5.0x10^-4 moles per - Brainly.com
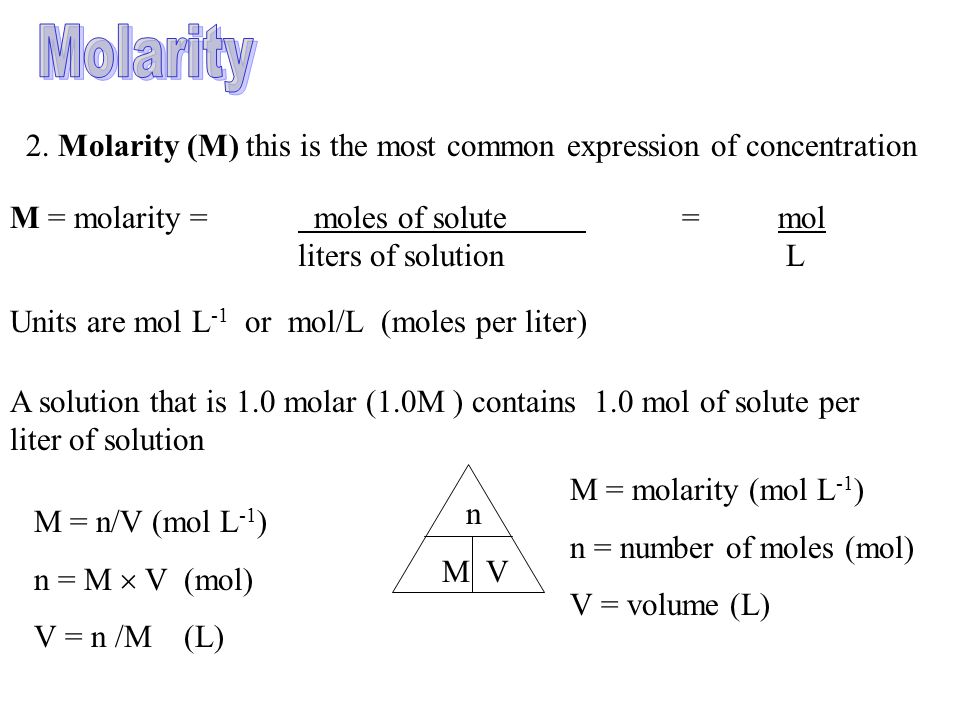
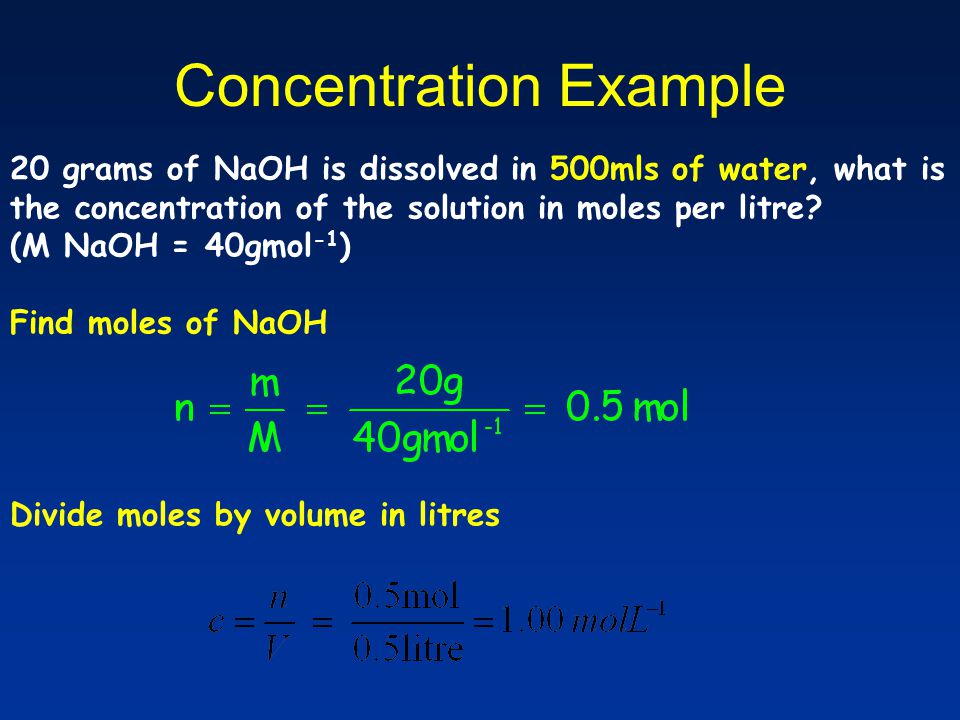
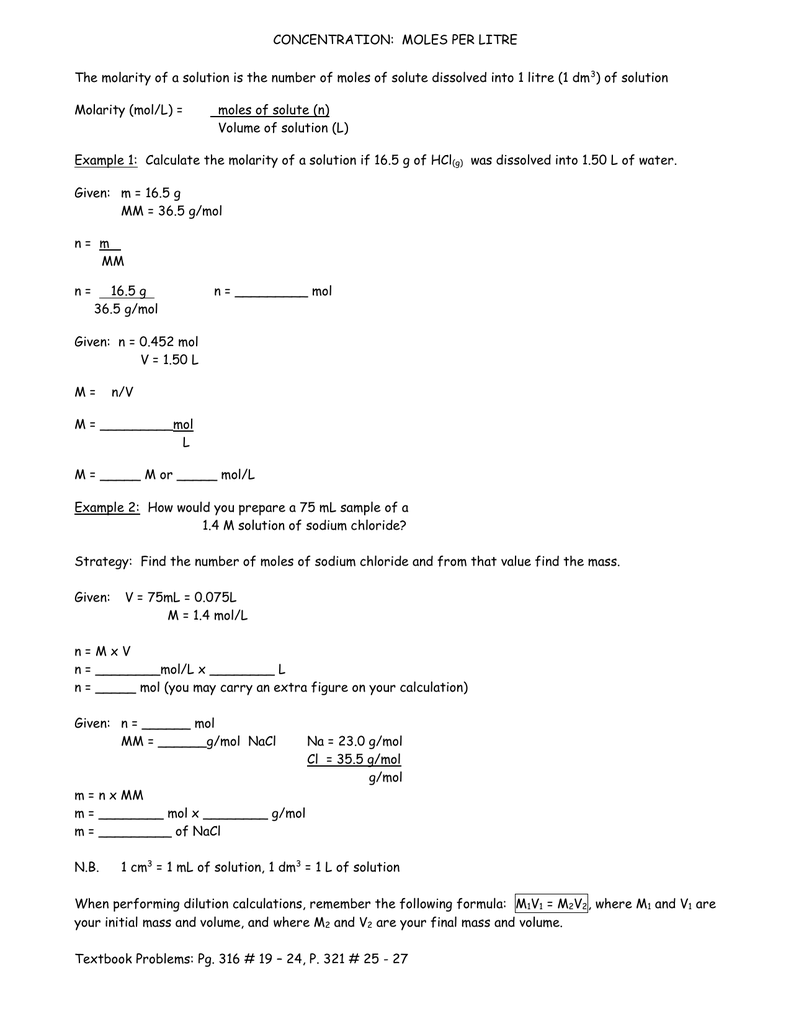
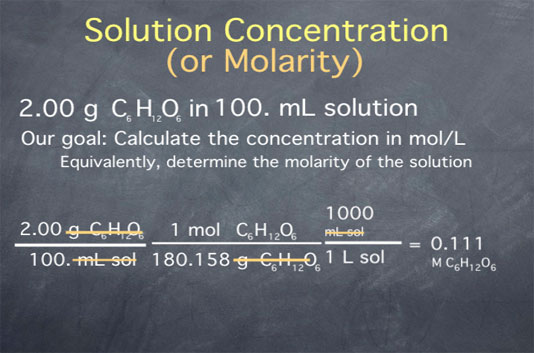
Molarity 2. Molarity (M) this is the most common expression of concentration M = molarity = moles of solute = mol liters of solution L Units are. - ppt download

Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL^-1 and the mass per cent of nitric acid in it being 69% .

Calculate the concentration of nitric acid in moles per litre in a sample which has density 1.41g/mL - YouTube
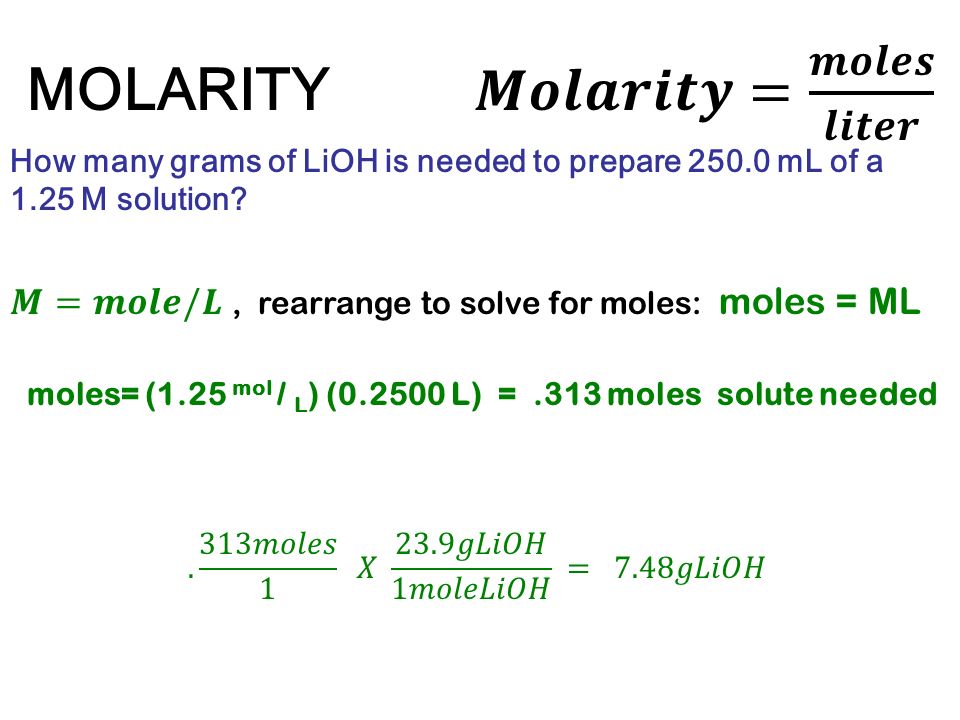
MOLARITY A measurement of the concentration of a solution Molarity (M) is equal to the moles of solute (n) per liter of solution M = mol / L Calculate. - ppt download