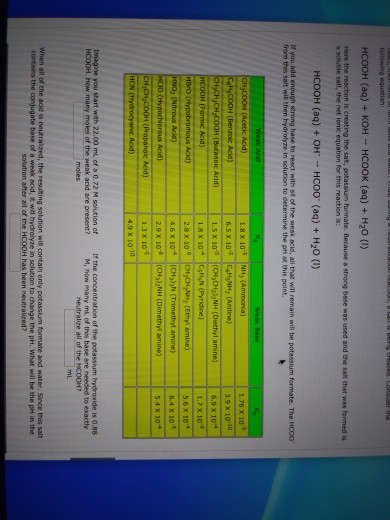
EHSQ (Environment,Health,Safety and Quality) : Question : Is HCOOK an acid or base or neutral ? Answer : HCOOK ( Potassium formate ) is base
In situ observation of [Mn-OOCH] by NMR. Reaction conditions: HCOOH (5... | Download Scientific Diagram
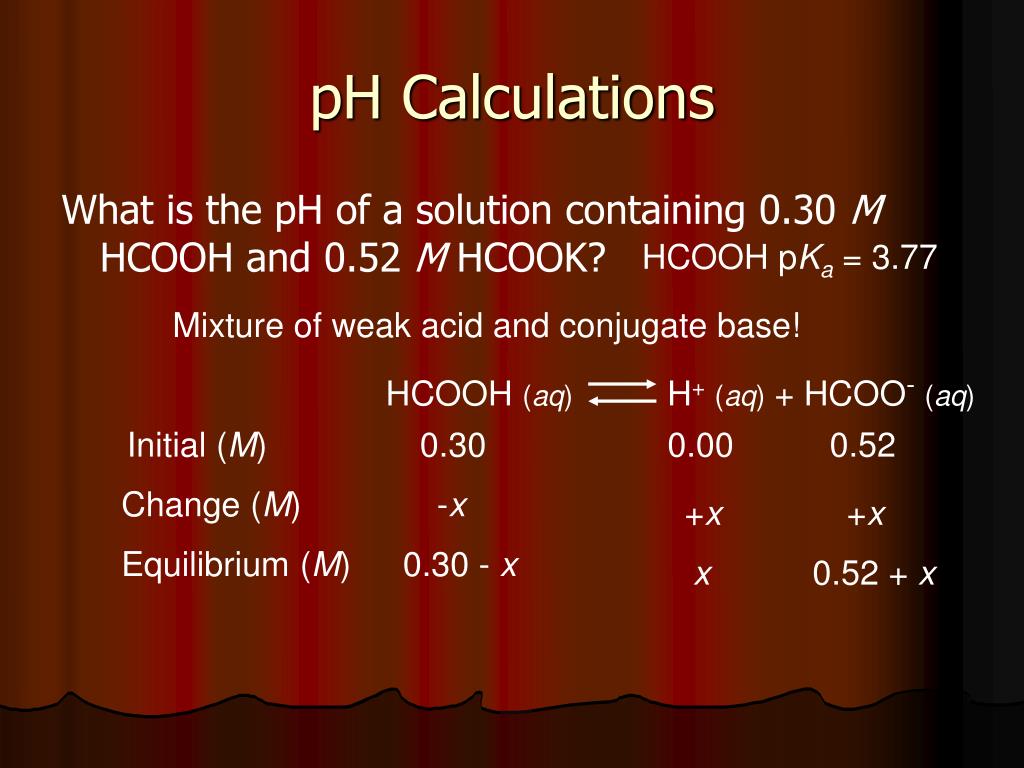
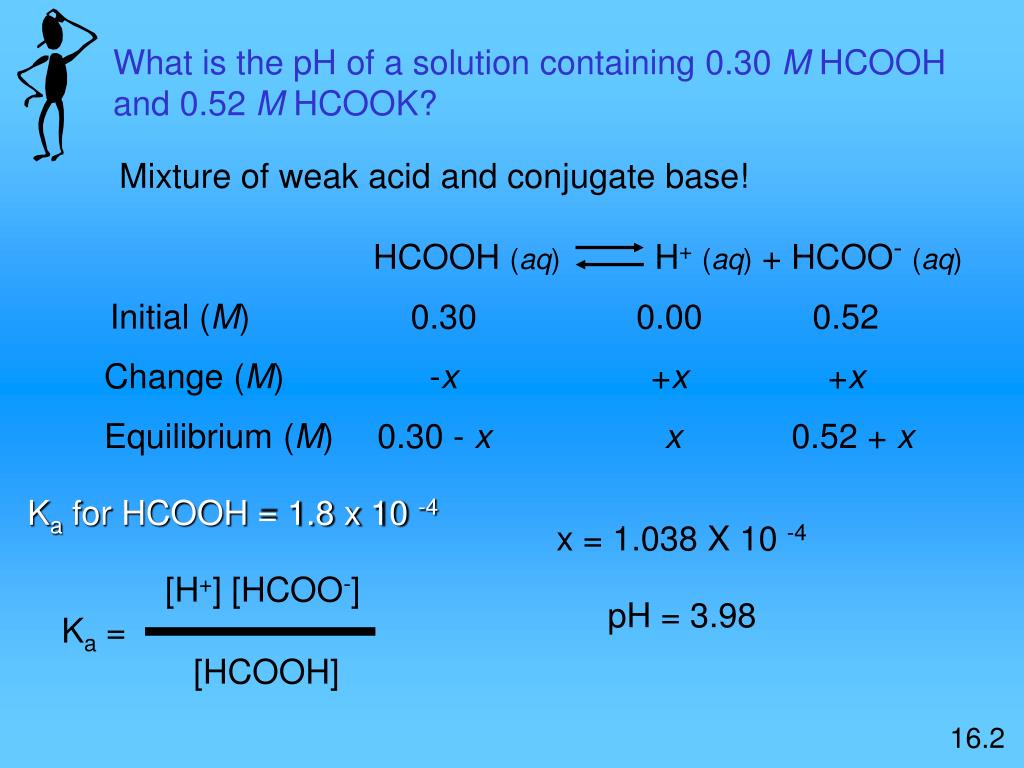
CHE 1302 Lecture Notes - Fall 2017, Lecture 12 - Equilibrium Constant, Hydrofluoric Acid, Rice Chart
![pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download](https://images.slideplayer.com/26/8472866/slides/slide_40.jpg)
pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download
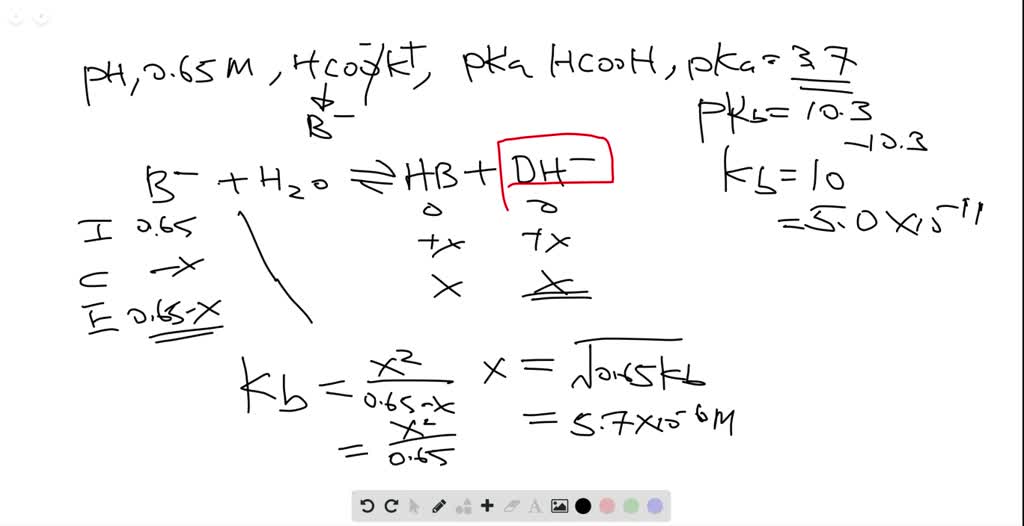
A 0.05M HCOOH and 0.1M HCOOK buffer solution was diluted 100 times. How did pH change and why? - Quora
Steady continuous dosage of FA. Reaction conditions: HCOOH (5 mmol),... | Download Scientific Diagram

Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl