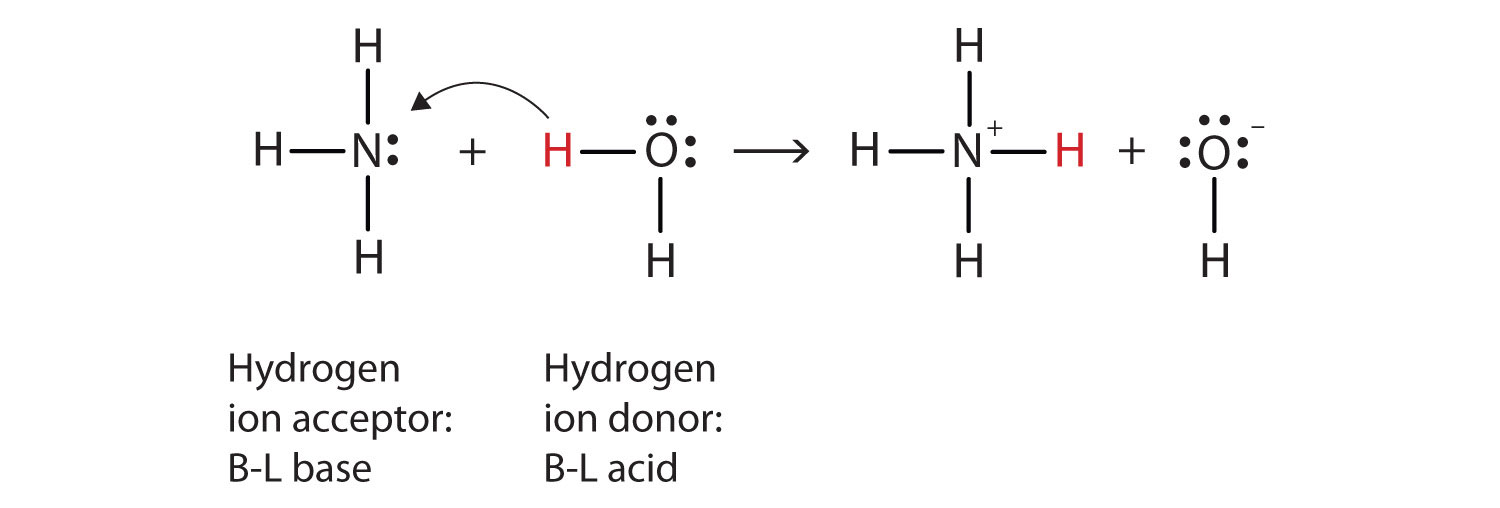
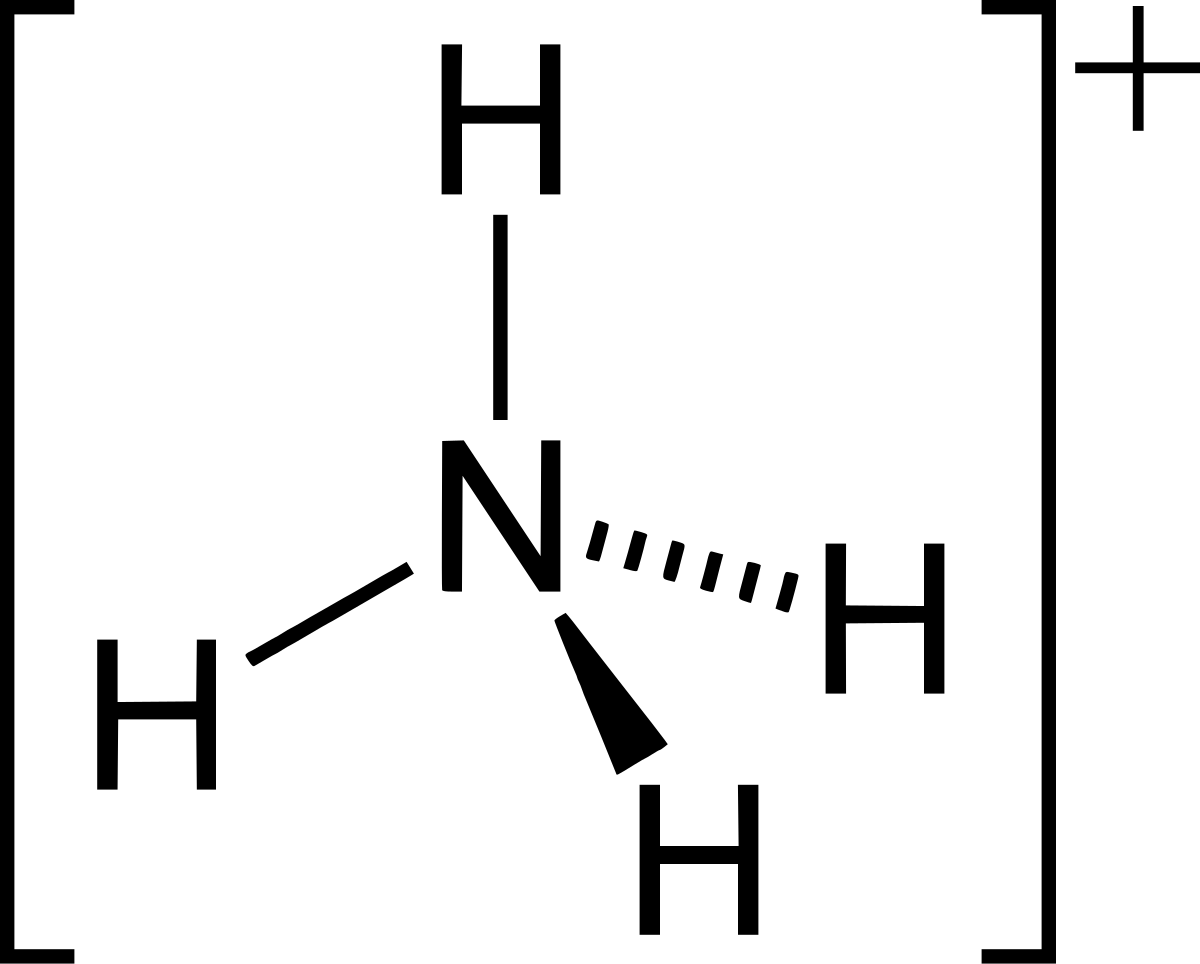
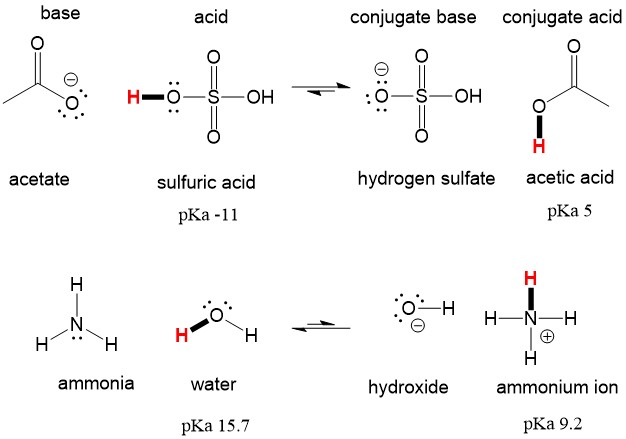
A novel method for molecular transformation to obtain energy from ammonia! | Nature Portfolio Chemistry Community

Question Video: Identifying the Lewis Acid in the Reaction of Ammonia with Boron Trifluoride | Nagwa

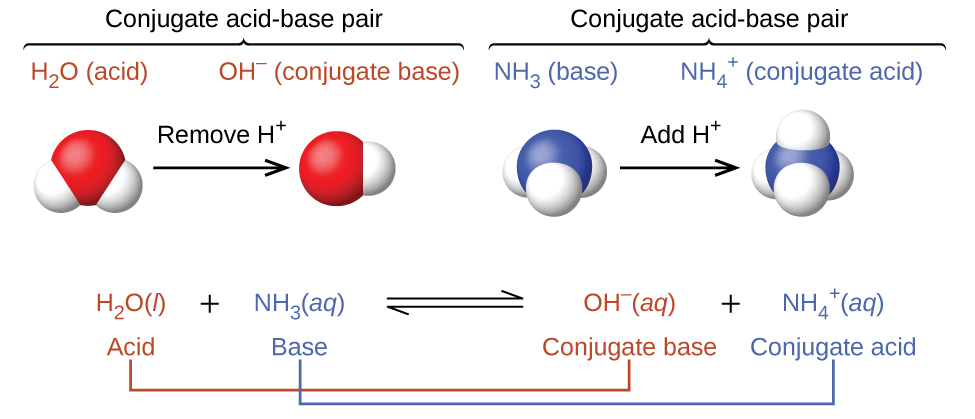
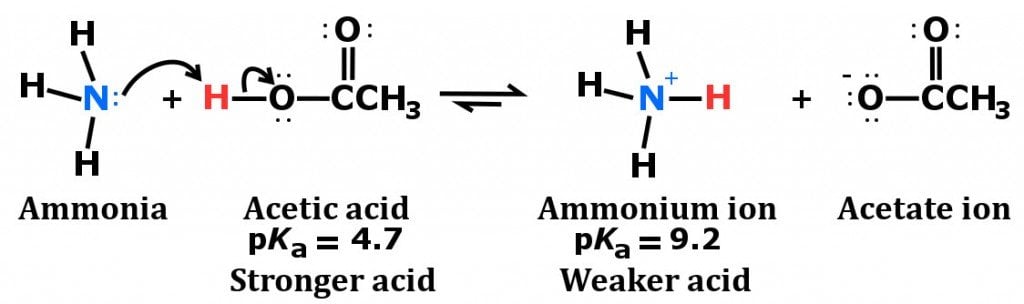
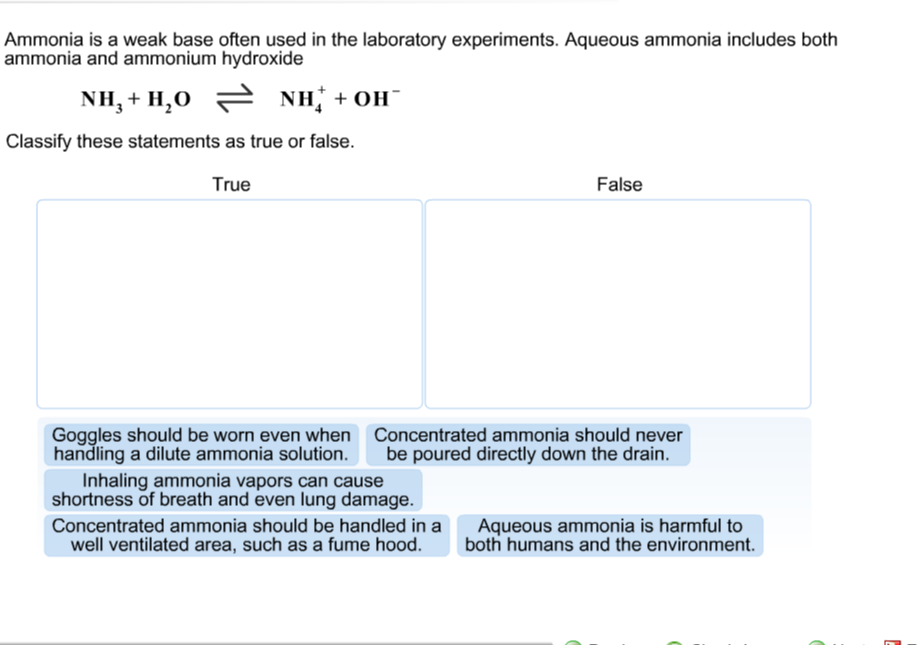
a) Explain why ammonia acts as a weak base in water. (b) Write a balanced chemical equation for the reaction between ammonia and water. | Homework.Study.com