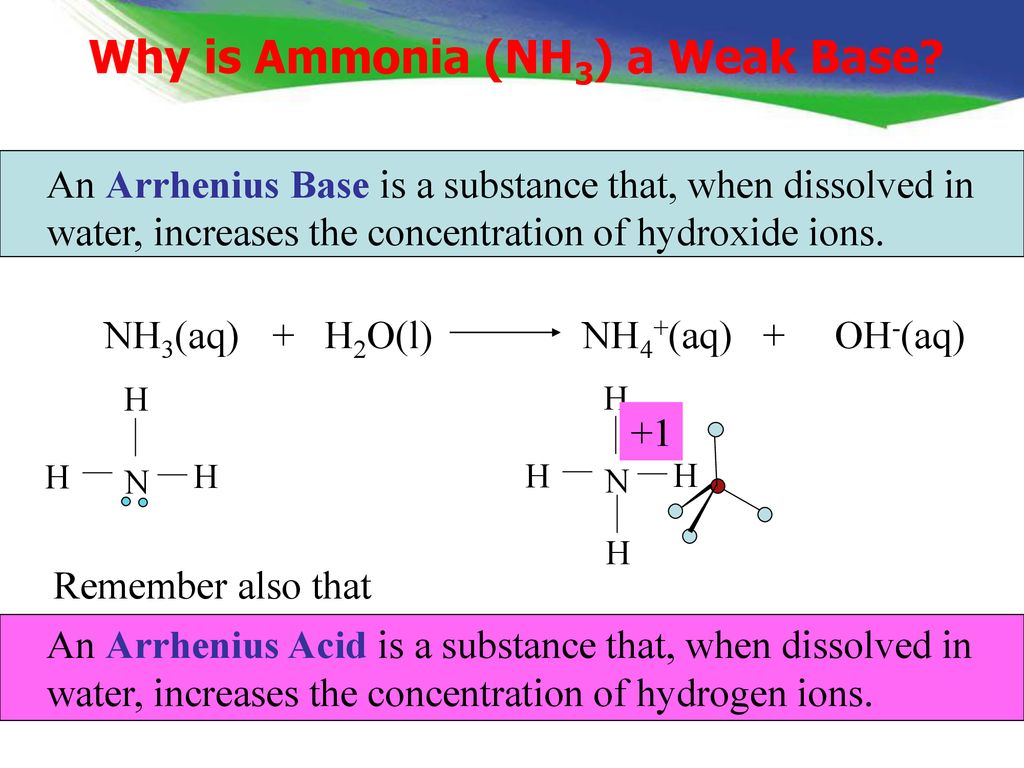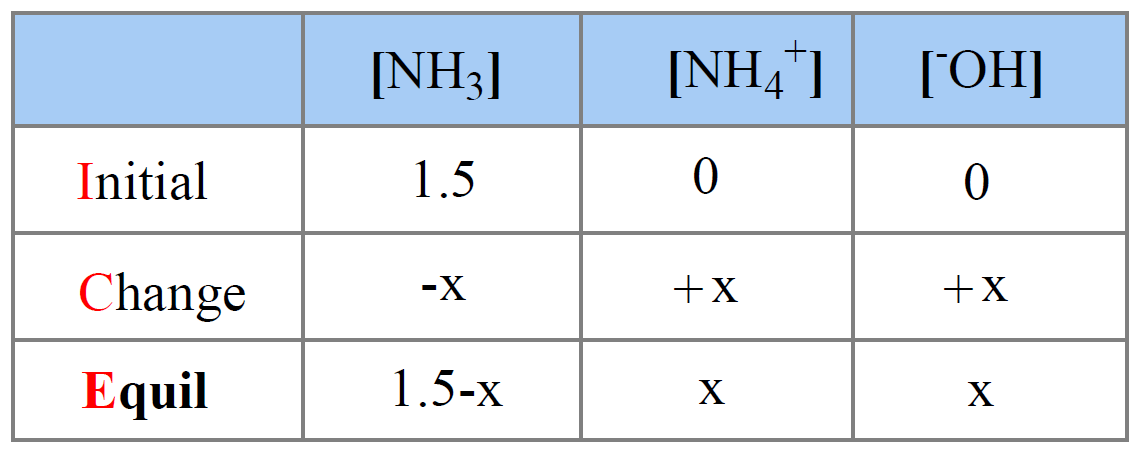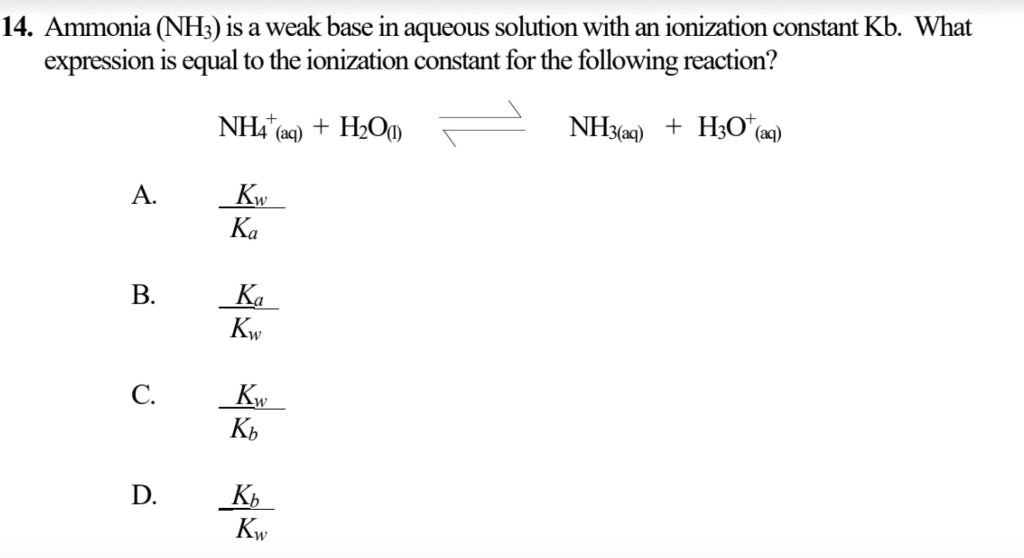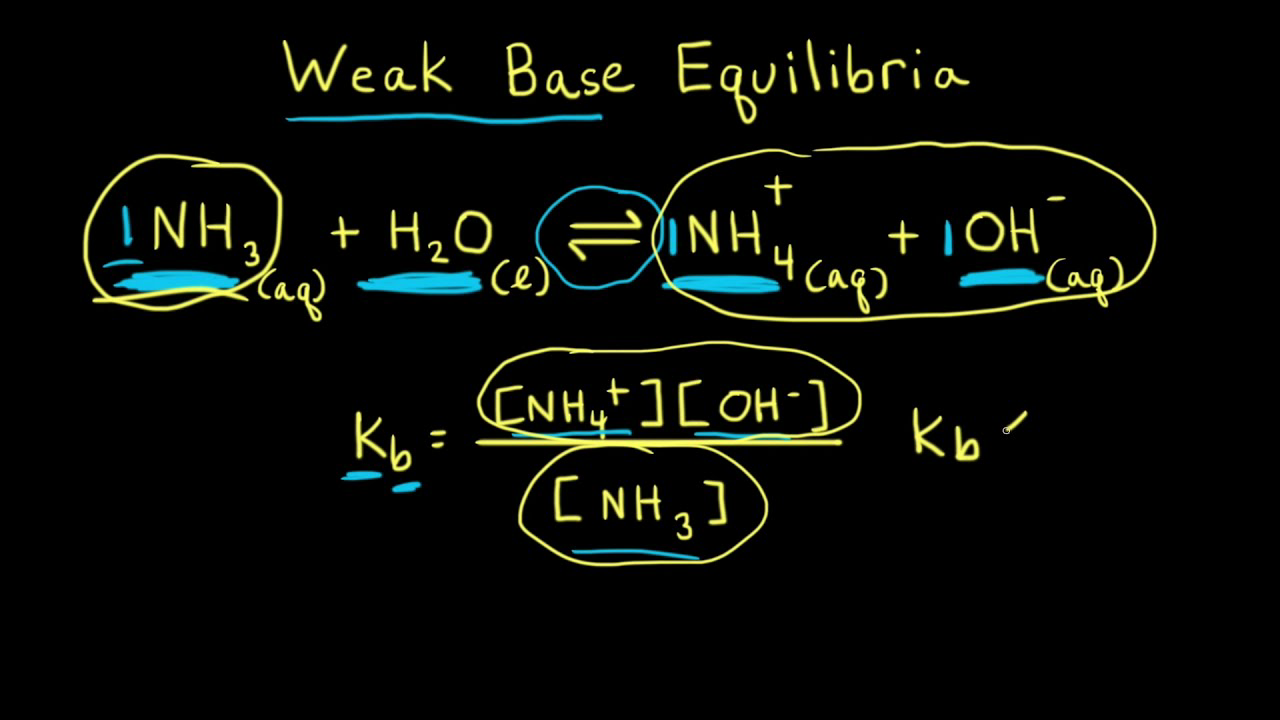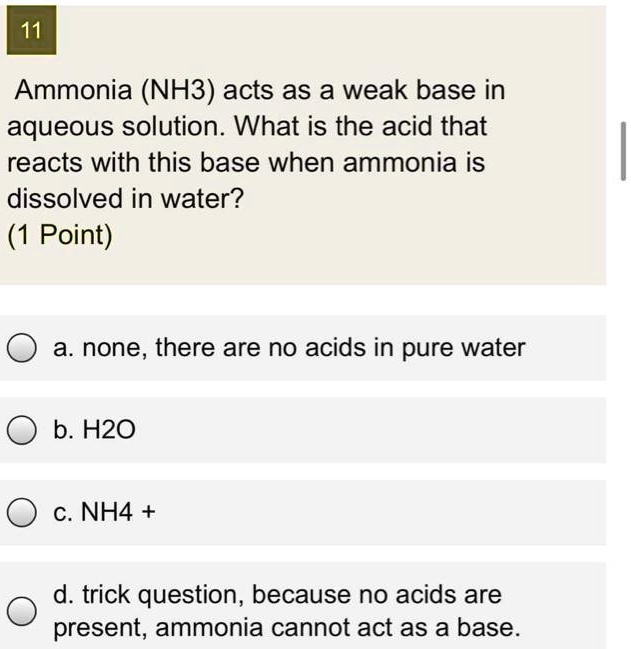
SOLVED: 11 Ammonia (NH3) acts as a weak base in aqueous solution. What is the acid that reacts with this base when ammonia is dissolved in water? (1 Point) none, there are

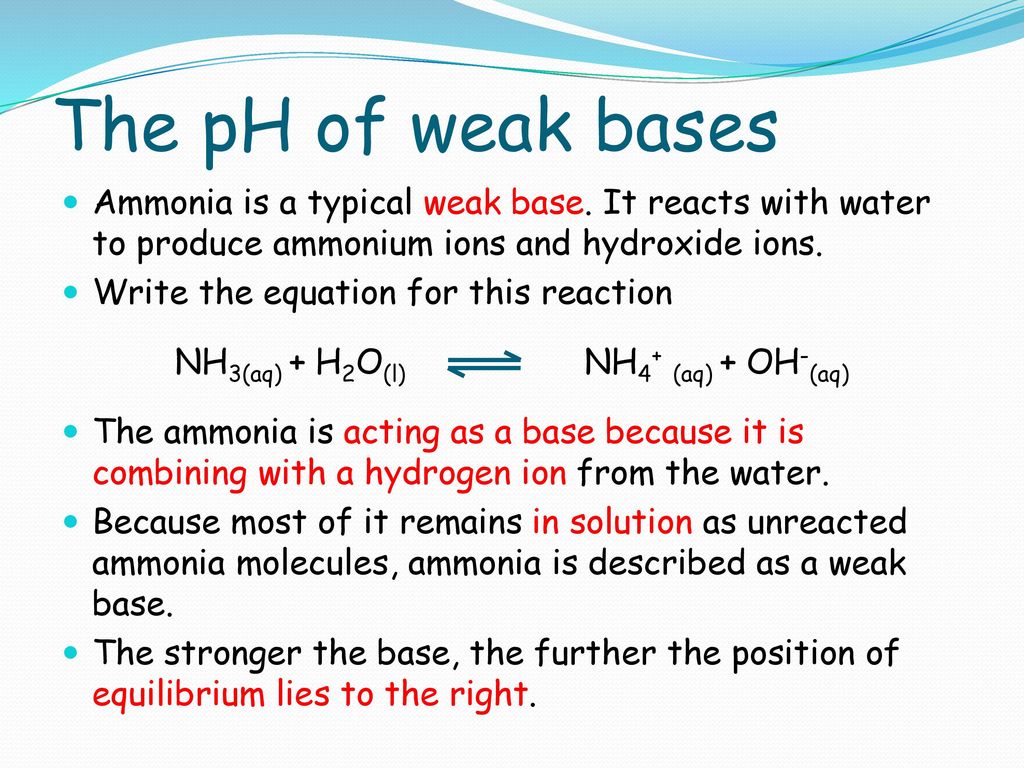
Ammonia is a weak base that reacts with water according to the equation: NH3(aq) + 2H2O(l) NH4^+(aq) + OH^-(aq) Which of the following conditions will decrease the moles of ammonium in water?

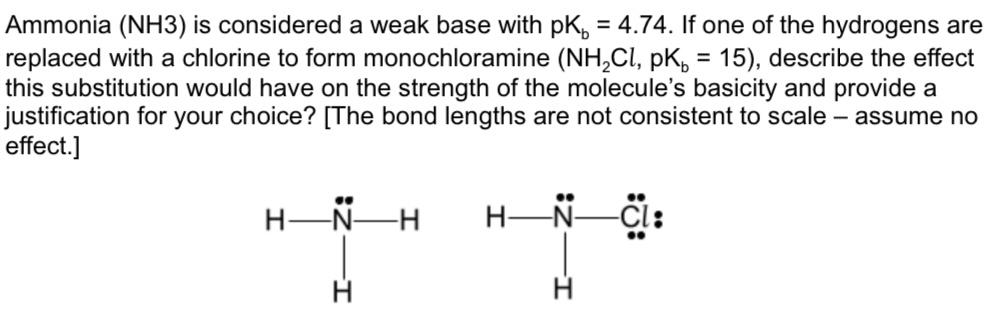
organic chemistry - Why In This Reaction Acetic Acid is strong acid and NH3 is strong base ?please explain in details and thanks for answer - Chemistry Stack Exchange

Ammonia is the weak base that reacts with water according to the equation: NH3(aq) + H2O(l)⇌NH4^ + (aq) + OH^ - (aq) Will any of the following increase the per cent of

NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in