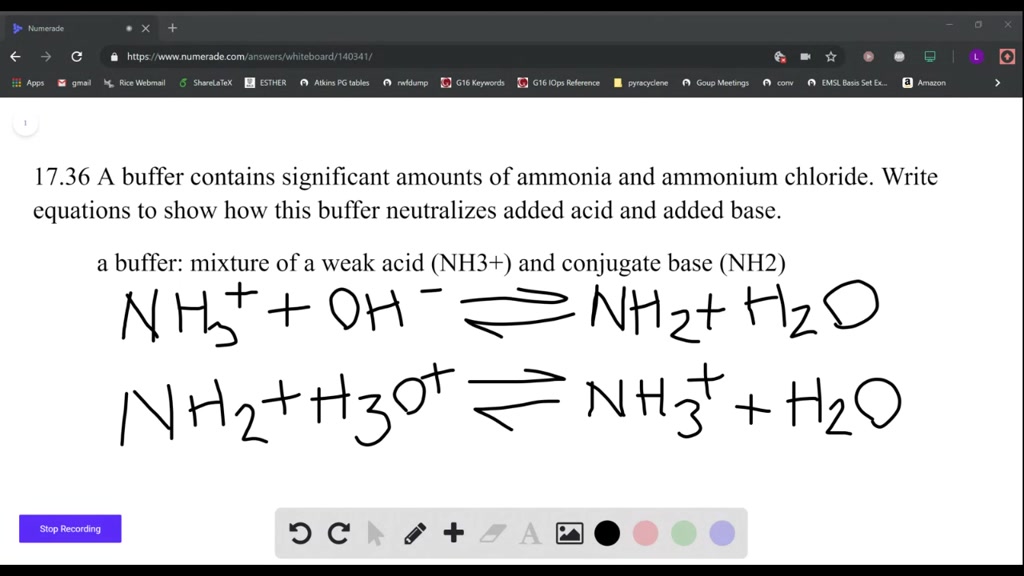
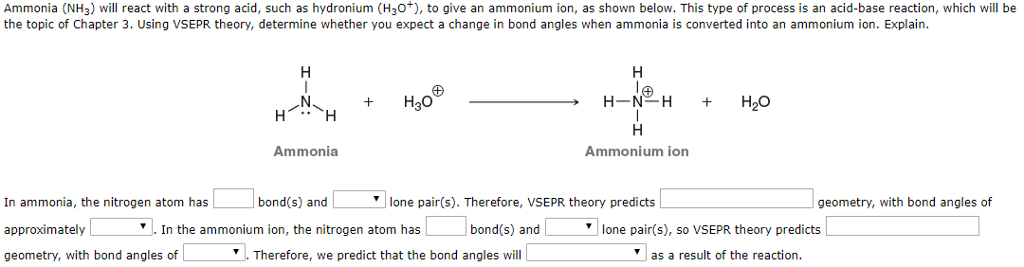
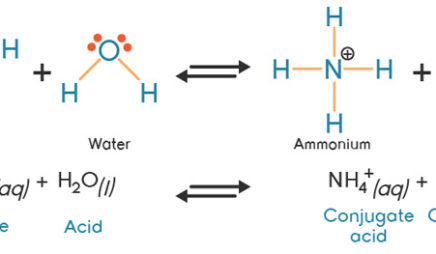
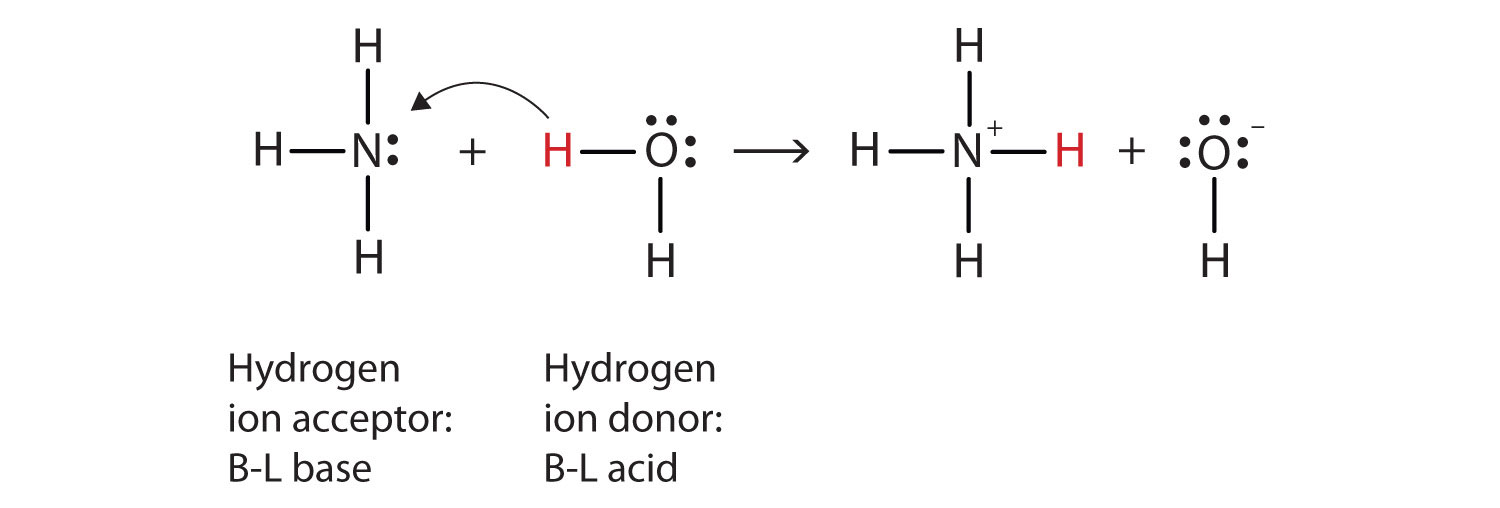
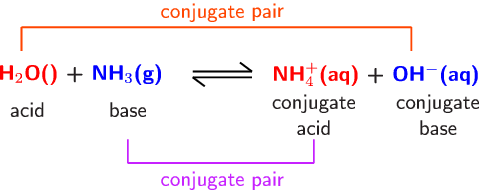
![high school : acid and base] is that ammonium ion a weak acid or base. Because it release OH- when react with water but when I search in Internet, it shows that high school : acid and base] is that ammonium ion a weak acid or base. Because it release OH- when react with water but when I search in Internet, it shows that](https://i.redd.it/kcgf74ac4i151.jpg)
high school : acid and base] is that ammonium ion a weak acid or base. Because it release OH- when react with water but when I search in Internet, it shows that

Write the predominant form for Butyl Ammonium is more water-soluble or more organically soluble. | Homework.Study.com

List of acids, bases and new ammonium-based protic ionic liquids (PILs)... | Download Scientific Diagram

On treatment with strong base quaternary ammonium ions R4N+, undergo elimination. However, the corresponding ammonium ions RNH3+ do not, although the basicity of NH3 is not very different from that of
Results of urea synthesis from ammonium carbamate catalyzed by organic... | Download Scientific Diagram
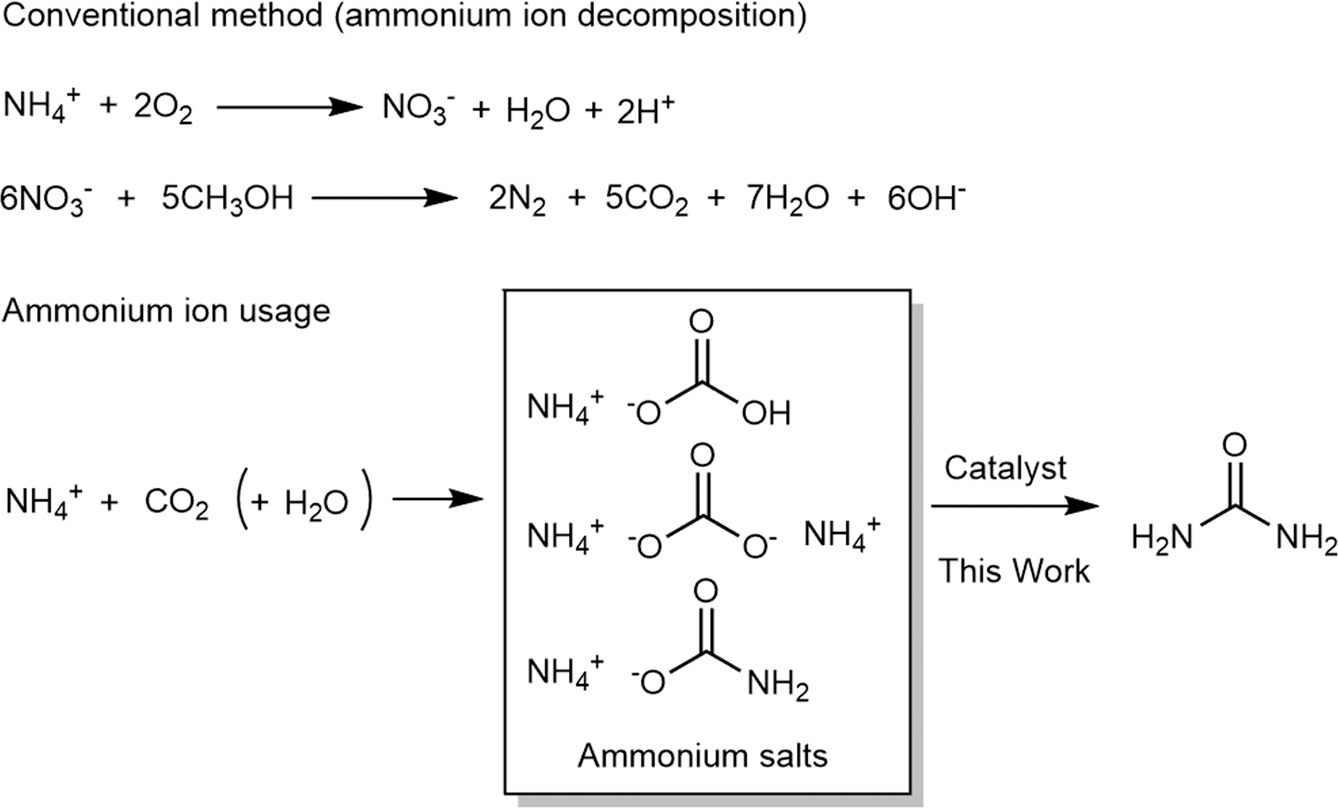
Organic bases catalyze the synthesis of urea from ammonium salts derived from recovered environmental ammonia | Scientific Reports