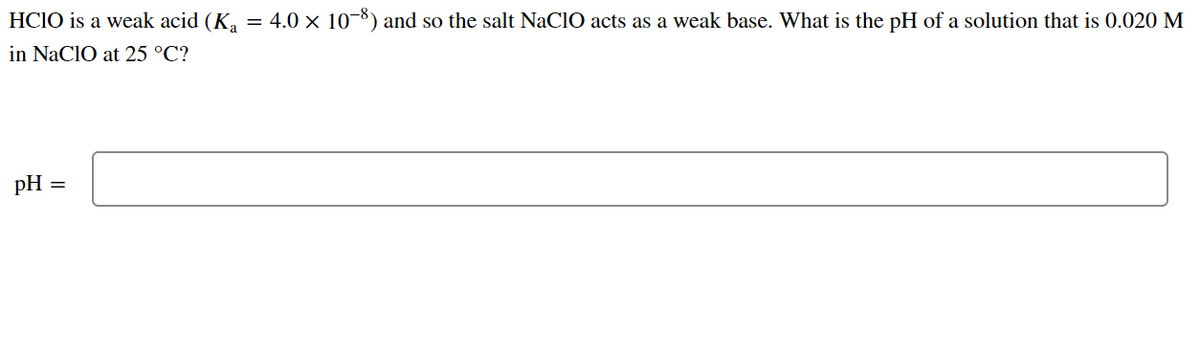
HClO is a weak acid (Ka = 4.0 x 10-8) and so the salt NaClO acts as a weak base. What is the pH of a solution that is 0.034 M in

HClO is a weak acid (Ka = 4.0 × 10–8) and so the salt NaClO acts as a weak base. What is the pH of a - Brainly.com
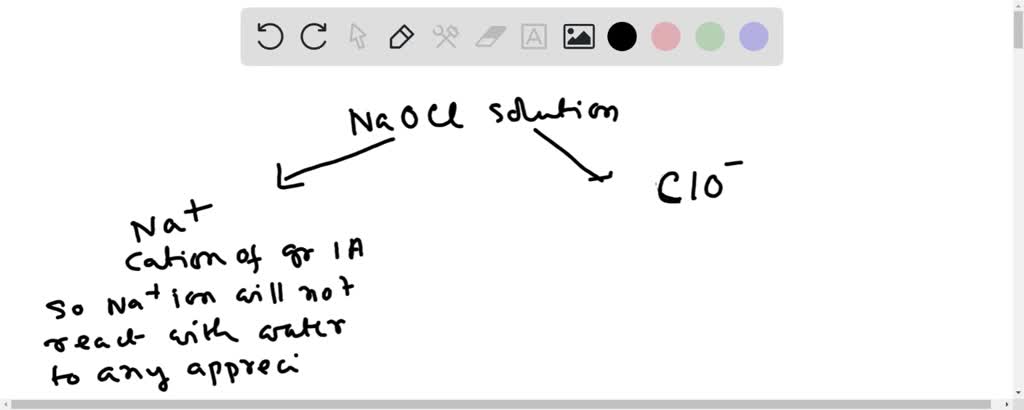
SOLVED: a) Why do salts from a strong base and a weak acid give a basic solution in aqueous solution? Use sodium hypochlorite (NaClO), used as a disinfectant, to illustrate this b)Why
OneClass: HClO is a weak acid (Ka = 4x10^-8) and so the salt NaClO acts as a weak base. What is the p...

Lab 24 - Hydrolysis A salt formed between a strong acid and a weak base is an acid salt. Ammonia is a weak base, and its salt with any strong acid gives. -
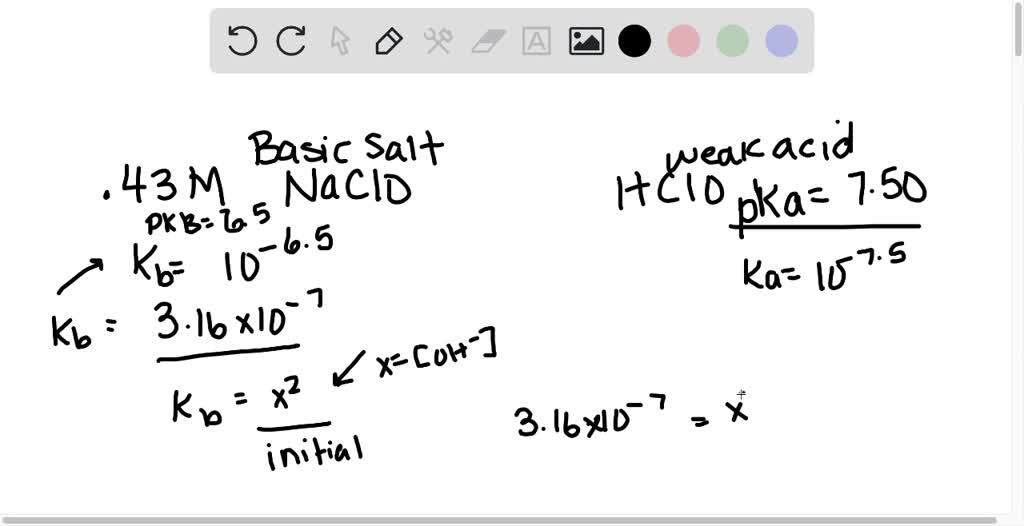


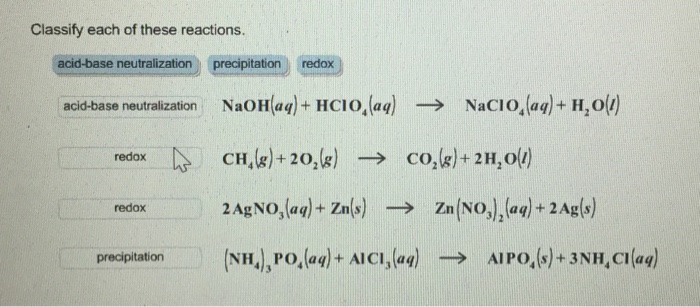







![Is NaOCl Acidic or Basic [Acids and Bases] - YouTube Is NaOCl Acidic or Basic [Acids and Bases] - YouTube](https://i.ytimg.com/vi/HXJWALr3BEY/maxresdefault.jpg)