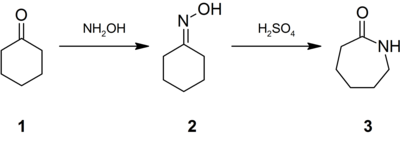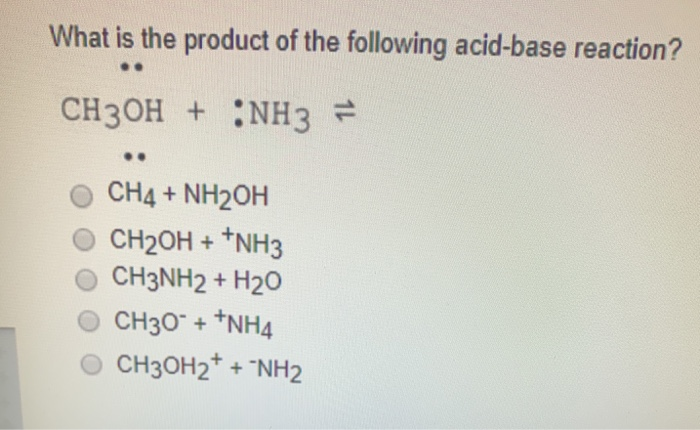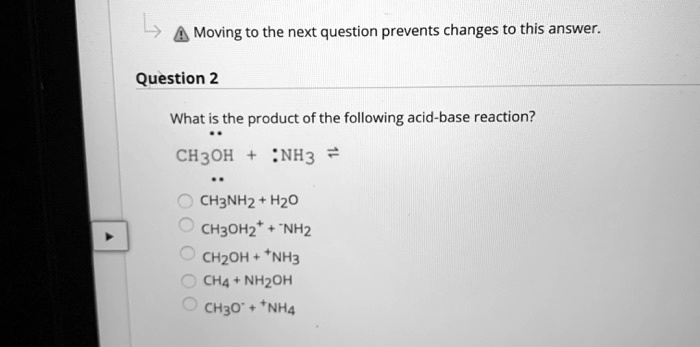
SOLVED: Moving to the next question prevents changes to this answer: Question 2 What is the product of the following acid-base reaction? CH3OH NH3 CH3NH2 Hzo CH30Hz* NH2 CH2OH "NH3 CHA NH2OH

OneClass: Label the Bronsted-Lowry acid and base as well as the conjugate acid and conjugate base for...

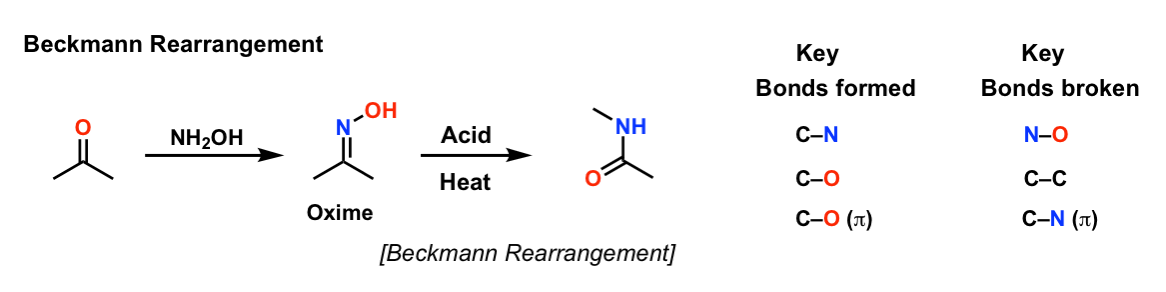
Photoorganocatalytic One‐Pot Synthesis of Hydroxamic Acids from Aldehydes - Papadopoulos - 2016 - Chemistry – A European Journal - Wiley Online Library

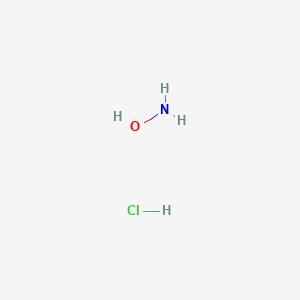
SOLVED: 1. Write the name of all strong acids and bases. 2. What is the difference between a strong and weak acid and bases? 3. Hydroxylamine (NH2OH) is a weak base with

Hydroxylamine as an Oxygen Nucleophile. Structure and Reactivity of Ammonia Oxide | Journal of the American Chemical Society

Palladium-catalyzed relay hydroaminocarbonylation of alkenes with hydroxylamine hydrochloride as an ammonia equivalent | Communications Chemistry

Calculate the pH of a 0.050 M solution of hydroxylamine, NH2OH. (Kb = 6.6 x 10-9) | Homework.Study.com
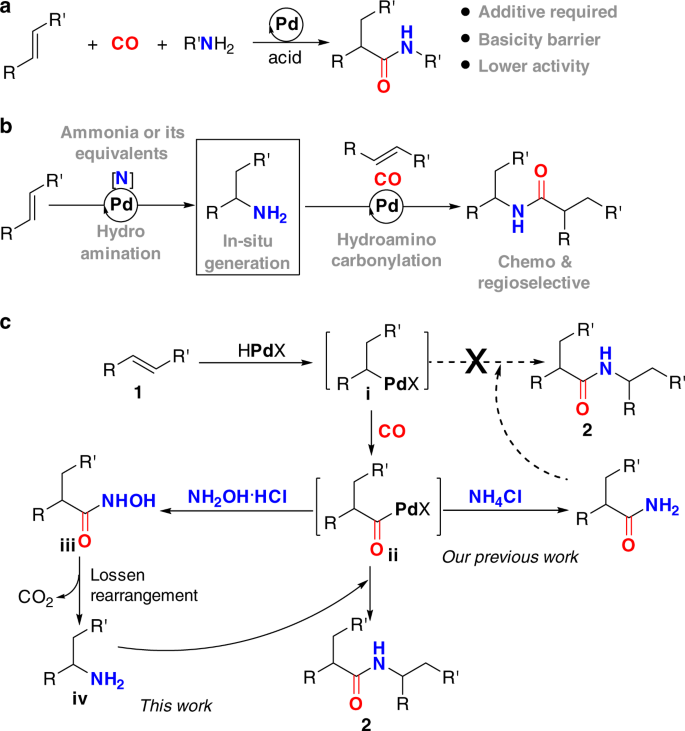
Palladium-catalyzed relay hydroaminocarbonylation of alkenes with hydroxylamine hydrochloride as an ammonia equivalent | Communications Chemistry
Synthesis of Hydroxamic Acids by Activation of Carboxylic Acids with N,N′-Carbonyldiimidazole: Exploring the Efficiency of the

OneClass: For the following reaction, K>1. Classify each of the reactants and products based on th...

Chapter 16. Overview: Definitions Arrhenius Bronsted -- Conjugate Pairs Hydronium Ion Relative Strengths Strong/Weak acids and reactions Strong/Weak bases. - ppt download